Electrode discovery points to simpler solar cell production method
Black silicon is silicon with a highly textured surface of nanoscale spikes or pores that are smaller than the wavelength of light. The texture allows the efficient collection of light from any angle, at any time of day. Barron and his team have been fine-tuning the creation of black silicon for some time, but an advance in the manufacturing technique should push it closer to commercialization, claimed Barron.
Barron said the work led by Rice postdoctoral researcher Yen-Tien Lu has two major attractions. “One, removing steps from the process is always good,” explained Barron. “Two, this is the first time in which metallization is a catalyst for a reaction that occurs several millimeters away.”
The metal layer used as a top electrode is usually applied last in solar cell manufacturing. The new method known as contact-assisted chemical etching applies the set of thin gold lines that serve as the electrode earlier in the process, which also eliminates the need to remove used catalyst particles.
The researchers discovered that etching in a chemical bath takes place a set distance from the lines. That distance, Barron said, appears to be connected to the silicon’s semiconducting properties.
“Yen-Tien was doing the reaction with gold top contacts, adding silver or gold catalyst and getting these beautiful pictures,” explained Barron. “And I said, ‘OK, fine. Now let’s do it without the catalysts.’ Suddenly, we got black silicon – but it was etching only a certain distance away from the contact. And no matter what we did, there was always that distance.

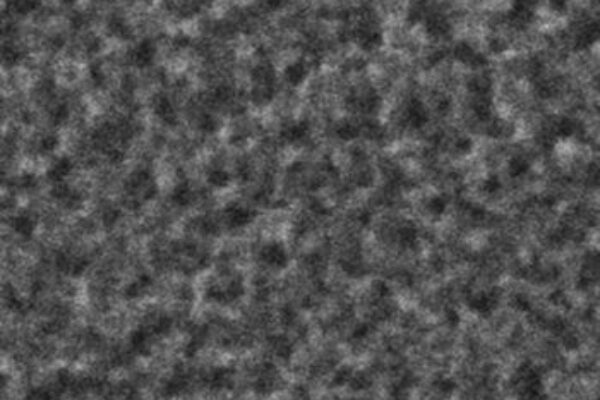
An electron microscope image shows fine, light-absorbing pores and spikes created in minutes on the surface of a silicon wafer for solar cells. Gold electrodes do double duty in the black silicon process developed by scientists at Rice University by serving as a catalyst to etch the surface in minutes. Image courtesy of the Barron Group
“It told us the electrochemical reaction is occurring at the metal contact and at the silicon that’s a certain distance away,” said Barron. “The distance is dependent upon the charge-carrying capacity, the conductivity, of the silicon. At some point, the conductivity isn’t sufficient for the charge to carry any further.”
An extremely thin layer of gold atop titanium, which bonds well with both gold and silicon, should prove to be an effective electrode that also serves for catalysis. “The trick is to etch the valleys deep enough to eliminate the reflection of sunlight while not going so deep that you cause a short circuit in the cell,” suggested Barron.
“Metal contacts are normally put down last,” said Barron. “It begs the question for a lot of processes of whether to put the contact down earlier and use it to do the chemistry for the rest of the process.”
The Rice lab of chemist Andrew Barron reported the research in the American Chemical Society journal ACS Applied Materials and Interfaces.
Related artices and links:
www.rice.edu
News articles:
Inkjet printing advance simplifies production of kesterite solar cells
Are hybrid perovskite field-effect transistors the future for solar cells?
Can tandem photovoltaics achieve record solar cell efficiencies?
 If you enjoyed this article, you will like the following ones: don't miss them by subscribing to :
eeNews on Google News
If you enjoyed this article, you will like the following ones: don't miss them by subscribing to :
eeNews on Google News




